Diamond Mineral Information And Data
Chemical Formula: C
Locality: Kimberly, republic of South Africa. India. Brazil. Ural Mountains, Russia. Murfreesboro, Arkansas, USA.
Name Origin: From the Greek, adamas, meaning “invincible” or “hardest.”
In mineralogy, diamond is a metastable allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at standard conditions. Diamond is renowned as a material with superlative physical qualities, most of which originate from the strong covalent bonding between its atoms. In particular, diamond has the highest hardness and thermal conductivity of any bulk material. Those properties determine the major industrial application of diamond in cutting and polishing tools and the scientific applications in diamond knives and diamond anvil cells.
Natural history
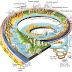
The formation of natural diamond requires very specific conditions—exposure of carbon-bearing materials to high pressure, ranging approximately between 45 and 60 kilobars (4.5 and 6 GPa), but at a comparatively low temperature range between approximately 900 and 1,300 °C (1,650 and 2,370 °F). These conditions are met in two places on Earth; in the lithospheric mantle below relatively stable continental plates, and at the site of a meteorite strike.
Physical Properties of Diamond
Cleavage: {111} Perfect, {111} Perfect, {111} Perfect
Color: Colorless, White, Gray, Black, Blue.
Density: 3.5 – 3.53, Average = 3.51
Diaphaneity: Transparent to Subtransparent to translucent
Fracture: Conchoidal – Fractures developed in brittle materials characterized by smoothly curving surfaces, (e.g. quartz).
Hardness: 10 – Diamond
Luminescence: Fluorescent and phosphorescent blue, Short UV=blue, Long UV=blue.
Luster: Adamantine
Streak: colorless











No comments
Post a Comment